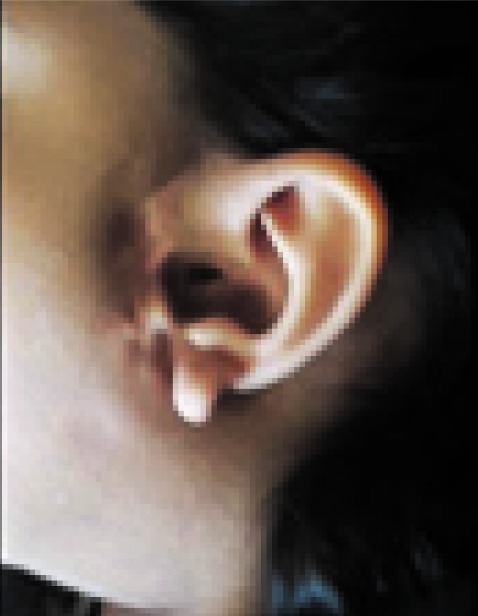
Goldenhar syndrome (GHS) is a complicated syndrome characterised by relation of mandibular hypoplasia, abnormality of the ear, ocular dermoid and vertebral issues and hemi facial macrosomia.
Treatment protocol is dependent upon the affected person’s age and systemic medical shows, with a multidisciplinary methodology typically being required.
This case report describes a typical 6-year-old feminine affected person who offered to the Department of Pediatric Dentistry, Faculty of Dentistry, University of Medical Sciences, Kerman, Iran with mandibular hypoplasia, facial asymmetry, ear tags and ocular dermoid after cosmetic surgery.
Diagnosis was primarily based on medical points, radiology and laboratory findings. GHS is a developmental criticism that may disturb many points of the affected person’s life; due to this fact, instant remedy from start is critical.
Breast most cancers sufferers’ return to work (B-CARE): protocol of a longitudinal mixed-methods research aiming to discover medical and occupational rehabilitation of sufferers with breast most cancers in Germany.
In latest years, analysis has been executed on determinants of return to work (RTW) in most cancers survivors and their long-term work outcomes. Nevertheless, little is understood in regards to the survivors’ analysis of these outcomes in phrases of job satisfaction and voluntariness.
Hence, B-CARE goals at filling the analysis hole by offering a longitudinal cohort research investigating medical and occupational rehabilitation together with an analysis by breast most cancers survivors.
A mixed-methods strategy, combining a quantitative survey with qualitative semi-structured interviews, is used to review breast most cancers survivors 5-6 years after prognosis.
These knowledge might be linked to knowledge from prior waves of sufferers throughout hospitalisation and 10 and 40 weeks after hospital discharge in addition to routine knowledge from the German Statutory Pension Insurance Scheme and German Cancer Society if obtainable.
The precise survey focuses on determinants of medical rehabilitation use, RTW, subsequent employment patterns put up care in addition to the voluntariness of and satisfaction with job adjustments.
A constructive vote from the ethics committee of the MedicalFaculty of the University of Bonn has been obtained. Data safety rules might be adhered to for all dealt with knowledge. Personal identifiers of contributors might be pseudonymised.
Dissemination methods embody a workshop to debate outcomes amongst stakeholders resembling representatives of the German Statutory Pension Insurance Scheme, social employees and self-help teams.German Clinical Trials Register (DRKS00016982);
